Unusual surface mass changes in the course of the oxygen reduction reaction on platinum and their explanation by using a kinetic model
The paper authored by
B.B. Berkes,
P.L. Simon,
K. Dobos,
Á. Nemes,
G. Inzelt and
Á. Kriston
is published in Journal of Solid State Electrochemistry (2012, vol. 16, pp. 1723–1732).
Abstract:
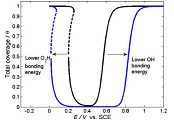
An unusual change of the surface mass with time has been observed during the oxygen reduction reaction on Pt using chronopotentiometry and simultaneous electrochemical quartz crystal nanobalance measurements. A simplified kinetic model of Damjanovic and Brusic, which involves two electrochemical and a chemical step, was analyzed using phase plane analysis. The theoretical analysis predicted that bistability might occur in this system at a certain set of parameter values. The mathematical simulation of the different trajectories explained well the strong influence of the starting potential and the current density on the change of the surface mass observed. Evidence was found that the surface coverage can increase at lower potentials, which can lead to the formation of hydrogen peroxide even if it is energetically unfavorable.
